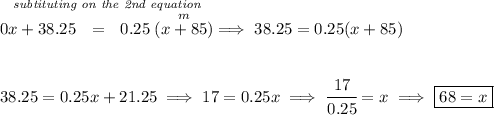so we're adding water to an iodine solution, how much iodine is in water? well, this is just plain water so it doesn't have any iodine, so 0%, let's say we'll add "x" ounces of water, to 85oz of 45% iodine to end up with an "m" ounces of a mixture at 25% iodine, so
![x=\textit{oz of solution of water at 0\%}\\\\ ~~~~~~ 0\%~of~x\implies \cfrac{0}{100}(x)\implies 0 \\\\\\ 85=\textit{oz of solution at 45\%}\\\\ ~~~~~~ 45\%~of~85\implies \cfrac{45}{100}(85)\implies 0.45 (85)\implies 38.25 \\\\\\ m=\textit{oz of solution at 25\%}\\\\ ~~~~~~ 25\%~of~m\implies \cfrac{25}{100}(m)\implies 0.25 (m) \\\\[-0.35em] ~\dotfill](https://img.qammunity.org/2024/formulas/mathematics/college/glveq5h9zl01z6iyb2ozlckgolh1wt33gw.png)
![\begin{array}{lcccl} &\stackrel{oz}{quantity}&\stackrel{\textit{\% of oz that is}}{\textit{iodine only}}&\stackrel{\textit{oz of}}{\textit{iodine only}}\\ \cline{2-4}&\\ \textit{1st Sol'n}&x&0.0&0x\\ \textit{2nd Sol'n}&85&0.45&38.25\\ \cline{2-4}&\\ mixture&m&0.25&0.25m \end{array}~\hfill \begin{cases} x + 85 = m\\\\ 0x+38.25=0.25m \end{cases} \\\\[-0.35em] ~\dotfill](https://img.qammunity.org/2024/formulas/mathematics/college/ft42q25yc8w5v84vj5ahdouotc8m58uty3.png)