Final answer:
For the endothermic reaction 2
 (g) +
(g) +
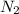 (g) → 2NO(g) + 2CO(g), high temperature will favor the formation of products, while pressure is unimportant. The correct option for conditions favoring maximum conversion to products is high temperature, pressure being unimportant.
(g) → 2NO(g) + 2CO(g), high temperature will favor the formation of products, while pressure is unimportant. The correct option for conditions favoring maximum conversion to products is high temperature, pressure being unimportant.
Step-by-step explanation:
The student has asked about the conditions that favor maximum conversion of reactants to products for an endothermic reaction. In this case, the reaction given is 2
 (g) +
(g) +
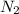 (g) → 2NO(g) + 2CO(g), and it is an endothermic process, which means that it requires heat to proceed. According to Le Chatelier's Principle, an increase in temperature will shift the equilibrium to the right for an endothermic reaction, favoring the formation of products.
(g) → 2NO(g) + 2CO(g), and it is an endothermic process, which means that it requires heat to proceed. According to Le Chatelier's Principle, an increase in temperature will shift the equilibrium to the right for an endothermic reaction, favoring the formation of products.
Next, considering pressure, we have to look at the number of moles of gas on each side of the reaction. Since the number of moles of gas molecules does not change (4 moles of gas reactants and 4 moles of gas products), changes in pressure have no significant effect on shifting the equilibrium. Thus, pressure is unimportant in this context.
Therefore, the conditions that favor maximum conversion of reactants to products for this reaction would be a high temperature, with the pressure being unimportant. This corresponds to option b from the choices provided.