Answer:
13%
Step-by-step explanation:
Mass percentage is used as a way to express the concentration of a solution, and is equal to the mass of the solute divided by the mass of the solution, which is then multiplied by 100%. This is express by the following equation: Mass percentage =
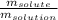 × 100%
× 100%
The mass of the solution is the sum of the individual masses of the solute and the solvent. The solute is the substance being dissolved, which in this case is glucose. The solvent is the substance (typically a liquid) capable of dissolving other substances to create a solution, and it is water in this problem.
Now that we've established the solvent and the solute, we can plug the value of their masses into the formula:
- Mass percentage =
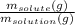 × 100%
× 100% - Mass percentage =
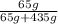 × 100%
× 100% - Mass percentage =
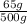 × 100%
× 100% - Mass percentage = 0.13 × 100%
- Mass percentage = 13%
Thus, the concentration of the solution in terms of mass by mass percentage is 13%.