The reduction of iron(III) oxide (Fe2O3) to iron (Fe) by carbon (C) is represented by the following chemical equation:
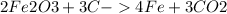
The temperature range at which carbon can reduce iron(III) oxide to iron depends on the thermodynamics and kinetics of the reaction. Let's break down the factors:
Thermodynamics: The reduction reaction will occur when the Gibbs free energy change (ΔG) is negative, indicating a spontaneous process. The Gibbs free energy change is influenced by the temperature and can be calculated using the equation:
ΔG = ΔH - TΔS
Where:
ΔG = Gibbs free energy change
ΔH = Enthalpy change
T = Temperature in Kelvin
ΔS = Entropy change
Kinetics: Even if the thermodynamics suggest a reaction is feasible, the rate at which it occurs also depends on temperature. Higher temperatures generally lead to faster reaction rates due to increased molecular motion.
The reduction of iron(III) oxide by carbon is typically carried out in a furnace or a similar high-temperature environment. The reaction is favored at elevated temperatures. Generally, the temperature range for this reduction process is between 800°C to 1200°C (1472°F to 2192°F).
At temperatures below this range, the reaction will be slow, and the iron production will be uneconomical. At temperatures significantly above this range, there may be issues with controlling the reaction and possible side reactions that could affect the quality of the final product.
It's important to note that the specific conditions and catalysts used in the reduction process may vary depending on the industrial application and the desired outcome. Also, practical considerations such as reaction time, product purity, and energy efficiency will also influence the choice of temperature range for this reduction reaction.