Answer:
24.59%
Step-by-step explanation:
Pre-Solving
We are given that 11.0 g of calcium metal reacted with water, and 5.00g of calcium hydroxide is produced.
We want to find the percent yield for this reaction.
First, we'll write the balanced chemical equation, then we'll use stoichiometry.
Solving
Chemical equation
First, let's write the chemical equation.
Ca (s) + H2O (l) --> Ca(OH)2 (aq) + H2 (g)
Now, we need to balance it.
Ca looks fine.
We have 4 hydrogens and 2 oxygens on the right side, while we only have 2 hydrogens and 1 oxygen on the left.
So, we put a 2 in front of H2O (l).
Our equation is now:
Ca (s) + 2H2O (l) --> Ca(OH)2 (aq) + H2 (g)
Stoichiometry
We'll start with our 11.0g of Calcium metal.
We need to convert it from grams to mols, then convert the calcium moles into moles of Ca(OH)2. We then take the moles of Ca(OH)2 and convert that into grams.
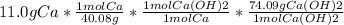 = 20.33g Ca(OH)2
= 20.33g Ca(OH)2
Percent Yield
The result we got from doing stoichiometry is our theoretical yield, while 5.00g of Ca(OH)2 given is our experimental yield.
To find the percent yield, we do
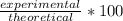
So, we do:
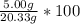 = 24.59%
= 24.59%