Answer:
0.163 grams (3 s.f.)
Step-by-step explanation:
To find the mass of oxygen collected, we first need to determine the partial pressure of oxygen in the collected gas. To do this, subtract the vapor pressure of water from the total atmospheric pressure to get the pressure exerted by the oxygen gas alone.
As 1 mm Hg = 1 Torr, then 762 mm Hg = 762 Torr.
Therefore:

To find the number of moles of oxygen gas, use the ideal gas law.
Ideal Gas Law
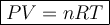
where:
- P is the pressure measured in torr (Torr).
- V is the volume measured in liters (L).
- T is the temperature measured in kelvin (K)
- R is the ideal gas constant (62.363598221529 L⋅Torr⋅K⁻¹⋅mol⁻¹)
- n is the number of moles.
Rearrange the equation to solve for n:
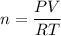
As we have been given the temperature in °C, we must first convert it to Kelvin by adding 273.15 to the Celsius value:
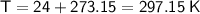
Therefore, the values to substitute into the formula are:
- P = 739.6 Torr
- V = 0.128 L
- T = 297.15 K
- R = 62.363598221529 L⋅Torr⋅K⁻¹⋅mol⁻¹
Substitute the values into the formula and solve for n:

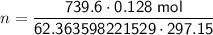
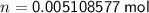
The molar mass of oxygen gas (O₂) is approximately 31.999 g/mol.
Therefore, to find the mass of oxygen collected, multiply the found number of moles (n) by the molar mass:

Therefore, the mass of oxygen collected is approximately 0.163 grams (3 s.f.).