To find the pH of a solution, it would be helpful to use this equation:
pH = - log [H]
*[H] will always be the molarity or concentration of a solution*
In this problem, you are given the pH, but not the molarity.
First, plug in each answer choice into the equation and solving with your calculator to see which equal 7. Let's try this:
- pH= - log (1 ×
 ) = -7
) = -7 - pH= - log( 1×
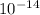 ) = 14
) = 14 - pH= -log ( 1 ×
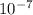 ) = 7
) = 7
According to this method, the answer would be C, 1 ×
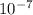 , since the answer was 7
, since the answer was 7