Answer:
B) 2.30 liters
Step-by-step explanation:
Given chemical equation:
Na₂O₂ + CO₂ → Na₂CO₃ + O₂
To solve this stoichiometry problem, we first need to balance the chemical equation.
To balance a chemical equation, we need to make sure that there are the same number of atoms of each element on both sides of the equation. We can do this by adding coefficients in front of a chemical symbol or formula where needed.
The balanced equation is:
2Na₂O₂ + 2CO₂ → 2Na₂CO₃ + O₂
The balanced equation shows a 2 : 1 molar ratio between CO₂ and O₂.
It means that for every 2 moles of CO₂ reacting, 1 mole of O₂ will be produced.
At STP (Standard Temperature and Pressure), 1 mole of any ideal gas occupies 22.4 liters.
Given the volume of CO₂ is 4.60 liters, the number of moles of CO₂ produced is:
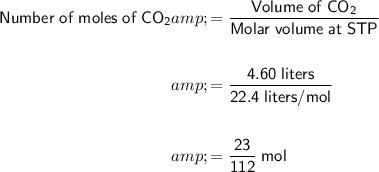
Since the stoichiometry is 2 : 1 between CO₂ and O₂, the number of moles of O₂ produced will be:
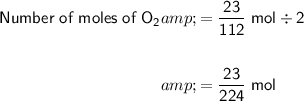
Finally, convert the moles of O₂ to volume at STP:
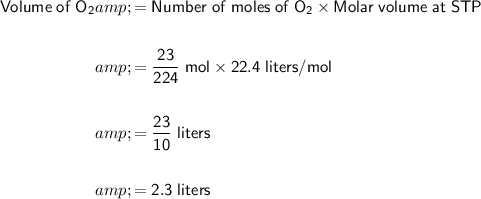
Therefore, the volume of O₂ gas produced is 2.30 liters.