The empirical formula, given that the molecular formula is
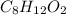 , is
, is
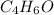 (option B)
(option B)
How to obtain the empirical formula when the molecular formula is given?
From the question above, we obtain the following data:
- Molecular formula of compound =
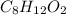
- Empirical formula of compound =?
From the molecular formula given in the question, we shall determine the common factor among the ratios of the various elements. This is shown below:
- C = 8
- H = 12
- O = 2
- Common factor =?
8 = 2 × 2 × 2
12 = 2 × 2 × 3
2 = 1 × 2
Common factor = 2
Now we shall divide the ratios of the various elements by two in order to obtain the empirical formula. Details below:
C = 8 / 2 = 4
H = 12 / 2 = 6
O = 2/2 = 1
Thus, the empirical formula is
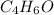 . Thus, the correct answer is option B
. Thus, the correct answer is option B