Answer:
Concentration of H+ ions at pH = 2=
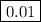
Concentration of H+ ions at pH = 6=
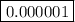
There are
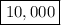 times more H+ ions in a solution at pH =2 and PH=6.
times more H+ ions in a solution at pH =2 and PH=6.
Step-by-step explanation:
The pH of a solution is defined as:
![\boxed{\tt pH = -log[H^+]}}](https://img.qammunity.org/2024/formulas/chemistry/high-school/dmm1rcqk97gid3vu39f5oa7xsayzya47c4.png)
where [H+] represents the concentration of H+ ions in mol/L.
In order to calculate the concentration of H+ ions at a given pH, we rearrange the formula:
![\boxed{\tt [H^+] = 10^(-pH)}](https://img.qammunity.org/2024/formulas/chemistry/high-school/ajpbiips56avmw6kwsi7eyzw23tnseeauv.png)
Now, let's calculate the concentration of H+ ions at pH = 2 and pH = 6:
1. Concentration of H+ ions at pH = 2:
![\tt [H^+] (pH = 2) = 10^(-2) \textsf{mol/L}](https://img.qammunity.org/2024/formulas/chemistry/high-school/fk3uwpbfcjkergs02hkkxna0w3sybbqsus.png)
2. Concentration of H+ ions at pH = 6:
![\tt [H^+] (pH = 6) = 10^(-6) \textsf{ mol/L}](https://img.qammunity.org/2024/formulas/chemistry/high-school/u08y4y8od07o4o1h2ra2z8jdeqd9yd4bji.png)
Now, to find how many more H+ ions there are in a solution at pH = 2 than in a solution at pH = 6, divide the concentration at pH = 2 by the concentration at pH = 6:
![\tt ([H^+] (pH = 2) )/( [H^+] (pH = 6) )= ((10^(-2) mol/L) )/((10^(-6) mol/L) )\\\\= 10^(-2 - (-6)) = 10^(-2 + 6)= 10^4](https://img.qammunity.org/2024/formulas/chemistry/high-school/xzgzbzgvaz9il5bhqxdeunm7ivvwq810ok.png)
So, there are 10,000 times more H+ ions in a solution at pH = 2 than in a solution at pH = 6.