Answer:
23.9 g = 0.0239 kg
Step-by-step explanation:
To determine the mass of lead oxide that reacts with 2,400 cm³ of carbon monoxide, we need to use stoichiometry and the balanced chemical equation for the reaction between lead oxide and carbon monoxide.
Lead oxide (PbO) consists of one lead atom and one oxygen atom.
Carbon monoxide (CO) consists of one carbon atom and one oxygen atom.
Metallic lead is obtained by reducing lead oxide with carbon monoxide.
The balanced chemical equation for this reaction is:
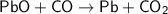
From the equation, we can see that the stoichiometric ratio between PbO and CO is 1 : 1. This means that for every 1 mole of PbO, 1 mole of CO is consumed.
At Standard Temperature and Pressure (STP), 1 mole of any gas will occupy a volume of 22.4 L. Therefore, convert the volume of CO to liters.
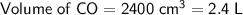
Convert the given volume of CO to moles at STP:
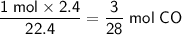
Since the balanced equation shows a 1 : 1 mole ratio between PbO and CO, the number of moles of PbO required is also 3/28 mol.
The molar mass of lead oxide (PbO) is 223.20 g/mol.
To calculate the mass of PbO, multiply the number of moles by the molar mass:
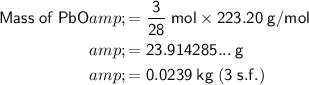
Therefore, approximately 23.9 grams of lead oxide (PbO) will react with 2,400 cm³ of carbon monoxide (CO) at STP.