Answer:
0.343 M
Step-by-step explanation:
To solve this problem, we can use the formula for dilution:
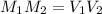
- where
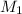 is the initial concentration of the solution,
is the initial concentration of the solution,
 is the initial volume of the solution,
is the initial volume of the solution,
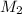 is the final concentration of the solution, and
is the final concentration of the solution, and
 is the final volume of the solution.
is the final volume of the solution.
Plugging in the given values, we get:
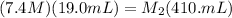
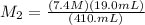
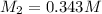
Therefore, the concentration of the solution formed by diluting
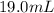 of a
of a
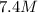 fructose solution to
fructose solution to
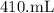 is
is
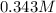 , rounded to three significant figures.
, rounded to three significant figures.