The image you sent shows four Lewis dot structures for the transfer of electrons between strontium and fluorine when they form an ionic compound. The correct answer is C.
![Sr^(+2)2[:\dot{F}^(-1)].](https://img.qammunity.org/2024/formulas/chemistry/college/32vijqoubr00d4lkluv2wfwtvnbv1ldku3.png)
Step-by-step explanation:
* Strontium has two valence electrons, while fluorine has seven.
* To form an ionic compound, strontium will transfer its two valence electrons to fluorine to form a full octet.
* This will result in strontium becoming a positively charged ion,
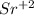 , and fluorine becoming a negatively charged ion,
, and fluorine becoming a negatively charged ion,
 .
.
* The Lewis dot structure for the ionic compound strontium fluoride,
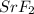 , is therefore
, is therefore
![Sr^(+2)2[:\dot{F}^(-1)]](https://img.qammunity.org/2024/formulas/chemistry/college/dzxatf4sr3tpevzaywieqfxjhjqe91579r.png) .
.
The other Lewis dot structures are incorrect:
* **A. $
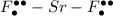 ** shows the atoms before they have formed an ionic compound, so the electrons are not distributed correctly.
** shows the atoms before they have formed an ionic compound, so the electrons are not distributed correctly.
* B. SE: shows a single bond between strontium and fluorine, but an ionic bond is formed by the transfer of electrons, not by sharing electrons.
* D.
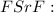 shows the atoms after they have formed an ionic compound, but the electrons are not distributed correctly.
shows the atoms after they have formed an ionic compound, but the electrons are not distributed correctly.