Problem 8:
Answer: b. 213.5 J
Step-by-step explanation:
Use the formula Q = mcΔT, where Q is heat, m is mass, c is specific heat, and ΔT is change in temperature.
Plug in values: Q = (0.015 kg)(2090 J/kg°C)(10°C) = 213.5 J
Problem 9:
Answer: a. 4010 J
Step-by-step explanation:
Use the formula Q = mL, where Q is heat, m is mass, and L is latent heat of fusion.
Plug in values: Q = (0.015 kg)(3.34 x 10^5 J/kg) = 4010 J
Problem 10:
Answer: c. 8279 J
Step-by-step explanation:
Use the formula Q = mcΔT (same as in Problem 8, but with water's specific heat).
Plug in values: Q = (0.015 kg)(4186 J/kg°C)(100°C) = 8279 J
Problem 11:
Answer: c. 33900 J
Step-by-step explanation:
Use the formula Q = mL, where Q is heat, m is mass, and L is latent heat of vaporization.
Plug in values: Q = (0.015 kg)(2.26 x 10^6 J/kg) = 33900 J
Problem 12:
Answer: d. 912 J
Step-by-step explanation:
Use the formula Q = mcΔT (same as in Problem 8, but with steam's specific heat).
Plug in values: Q = (0.015 kg)(1520 J/kg°C)(40°C) = 912 J
Problem 13:
Answer: d. 76414.5 J
Step-by-step explanation:
Add up the heat amounts from Problems 8-12 to get the total heat required: 213.5 J + 4010 J + 8279 J + 33900 J + 912 J = 76414.5 J
Complete Question:
In Problems 8-13 we will solve the following problem step by step: how much heat is need to convert
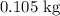 of ice at
of ice at
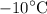 to steam at
to steam at
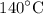 . The specific heat of ice is
. The specific heat of ice is
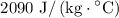 , the latent heat of fusion of water is
, the latent heat of fusion of water is
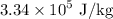 , the specific heat of water is
, the specific heat of water is
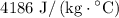 , the latent heat of vaporization of water is
, the latent heat of vaporization of water is
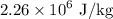 , and the specific heat of steam is
, and the specific heat of steam is
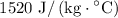 .
.
Problem 8:
How much heat is needed to increase the temperature of
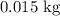 of ice from
of ice from
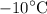 to
to
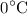 ?
?
a. 113.5 J
b. 213.5 J
c. 313.5 J
d. 413.5
Problem 9:
How much heat is needed to convert
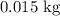 of ice at
of ice at
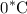 to
to
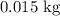 of water at
of water at
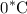 ?
?
a. 4010 J
b. 5010 J
c. 6010 J
d. 7010 J
Problem 10:
How much heat is needed to increase the temperature of
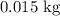 of water from
of water from
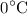 to
to
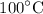
a. 6279 J
b. 7279 J
c. 8279 J
d. 9279 J
Problem 11:
How much heat is needed to convert
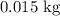 of water at
of water at
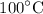 to
to
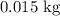 of steam at
of steam at
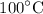 ?
?
a. 13900 J
b. 23900 J
c. 33900 J
d. 43900 J
Problem 12:
How much heat is needed to convert
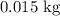 of steam at
of steam at
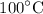 to
to
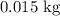 of steam at
of steam at
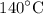 ?
?
a. 612 J}
b. 712 J
c. 812 J
d. 912 J
Problem 13:
How much heat is need to convert
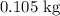 of ice at
of ice at
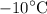 to steam at
to steam at
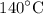 ? Use your answers from Problems 8-12.
? Use your answers from Problems 8-12.
a. 46414.5 J
b. 56414.5 J
c. 66414.5 J
d. 76414.5 J