Answer:
The mass of N2O5 is 108.0 g
The mass of Fe(ClO)2 is 158.7 g
The mass of Fe(ClO3)2 is 222.7 g
Step-by-step explanation:
Use this formula to calculate the mass of a compound:
number of moles=
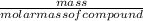 or in shorter form: n=
or in shorter form: n=
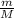 .
.
Since you're given the number of moles and the molar mass of each compound, simply plug those values into the formula above and solve for the mass (mass=number of moles x molar mass of compound).
Sample calculation for the mass of N2O5:
Molar mass=108.0 g/mol
Number of moles=1 mol.
Mass=?
Mass=number of moles x molar mass of compound
Mass=1 mol. x 108.0 g/mol.
Mass=108.0 g