Answer: 40.0 grams of Ar
Step-by-step explanation:
Using the formula
 , we see that
, we see that
 and
and
 are proportional, so we want the option with the most moles.
are proportional, so we want the option with the most moles.
- The atomic mass of Ar is 39.95 g/mol, so this sample has 1.001 moles
- This sample has 0.108 moles.
- Using Avogadro's number, this sample has
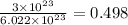 moles.
moles.