The number of molecules in 4.00 mol H₂O is 2.41 × 10²⁴ molecules (Option A).
How can the number of molecules be calculated?
A molecule is a collection of two or more atoms bound together by chemical bonds; depending on the context, ions that meet this requirement may or may not be included in the phrase.
Given;
4.00 mol of H₂O
Avogadro's number= 6.02 × 10²³ particles per mole.
The total number of molecules=
4.00 mol H₂O × (6.02 ×
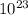 molecules/mol)
molecules/mol)
= 2.41 × 10²⁴ molecules.
Therefore, the correct answer is A. 2.41 ×
 molecules.
molecules.