The atomic mass of x is approximately 30.62 amu.
Define the isotopic abundances and masses:
Abundance of
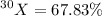
Abundance of
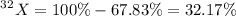
Mass of
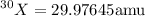 (atomic mass units)
(atomic mass units)
Mass of

Calculate the weighted average atomic mass:
The atomic mass is a weighted average of the individual isotope masses, where the weights are the corresponding isotopic abundances. We can use the following formula:
Average atomic mass = (abundance of isotope 1 × mass of isotope 1) + (abundance of isotope 2 × mass of isotope 2) + ...
In this case, the formula becomes:
Average atomic mass = (0.6783 × 29.97645 amu) + (0.3217 × 31.97381 amu)
Calculating this, we get:
Average atomic mass ≈ 30.6193 amu
Round to 4 significant figures:
Since we were given the abundances to 4 significant figures, the final answer should also be reported to 4 significant figures. Therefore, the rounded atomic mass of element X is:
Average atomic mass ≈ 30.62 amu