Final Answer:
(a) The equilibrium temperature of the system is 0.0°C.
(b) There is no mass of unmelted ice when the system is at equilibrium.
Step-by-step explanation:
When three ice cubes at 0°C are added to water at 24.0°C, heat transfer occurs until thermal equilibrium is reached. The heat gained by the ice as it melts equals the heat lost by the water as it cools down. Using the principle of conservation of energy, the final temperature is calculated using the formula:
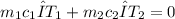
Where
 and
and
 are the masses of ice and water respectively,
are the masses of ice and water respectively,
 and
and
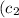 are their specific heats, and ΔT is the change in temperature.
are their specific heats, and ΔT is the change in temperature.
The heat released by the water is equal to the heat absorbed by the ice to melt and warm up to the final equilibrium temperature. At equilibrium, all the ice melts, leaving no unmelted ice. Therefore, the final answer for the equilibrium temperature is 0.0°C, and there's no remaining mass of unmelted ice.
This calculation assumes no heat exchange with the surroundings and neglects any temperature change in the container itself. The equilibrium temperature represents the point at which both the ice and water have exchanged heat to reach a common final temperature, resulting in complete melting of the ice and no remaining unmelted ice in the system.