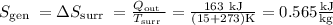(a) Final pressure,
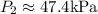
(b) Heat transfer,
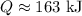
(c) Entropy generation,
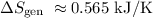
a) The final pressure in the tank can be determined by using the steam tables to determine the properties of the saturated liquid at the initial state and for the compressed liquid at the final state.
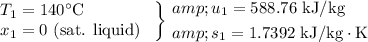
Since we want a zero entropy change,
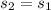 and therefore the final state properties are:
and therefore the final state properties are:
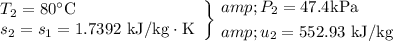
b) The heat transfer between the tank and the surroundings can be determined from and energy balance on the system, with the contents of the tank being the system:
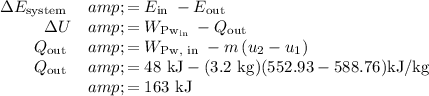
c) The entropy generation for this process will only be the result of the entropy change of the surroundings, since the entropy change of water is zero. This gives: