Answer:
The mass of the potassium aluminum sulfate dodecahydrate is heavier than the mass of the aluminum foil due to the difference in their chemical compositions and molecular structures.
Step-by-step explanation:
Aluminum foil consists solely of aluminum atoms, with a molar mass of 26.99 g/mol. On the other hand, potassium aluminum sulfate dodecahydrate, commonly known as alum, has a more complex chemical formula:
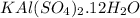 .
.
In this compound, potassium (K), aluminum (Al), sulfur (S), oxygen (O), and hydrogen (H) are all present. Additionally, there are 12 water molecules (H2O) associated with each unit of the compound.
The molar mass of potassium aluminum sulfate dodecahydrate is significantly higher at 474.39 g/mol due to the additional atoms and water molecules present in its structure. The water molecules contribute significantly to the overall mass, as water has a molar mass of 18.02 g/mol.
Therefore, when comparing the mass of the aluminum foil (reactant) to the mass of the potassium aluminum sulfate dodecahydrate (product), the latter is much heavier due to the inclusion of various atoms and water molecules in its composition.