a) The energy of the photon is 2.3210³ eV.
b) The photon energy is 4.7610⁵ eV.
To find the energy of a photon, we can use the following formula:
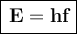
Where
- E is the energy
- h is planck's constant
- f is the frequency
a) If the frequency of the button is 5.60 10¹⁷ Hz, then its energy is:
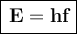
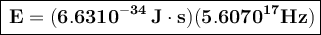
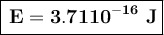
To convert this energy to units of electron volts, we divide by the value of electron volts:

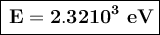
The energy of the photon is 2.3210³ eV.
b). If the wavelength is 2.6010^-2 nm, then we can use the relationship between wavelength and frequency.
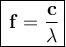
Where:
- C is the speed of light
- λ is the wavelength
The frequency of the photon is then:
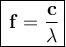
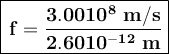
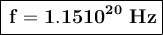
Using the energy formula we have, we have:
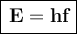
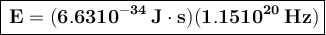
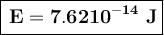
To convert this energy to units of electron volts, we divide by the value of electron volts:

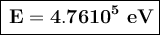
The photon energy is 4.7610⁵ eV.