Required Answer:
- the molarity of the solution is 1.2 M
Step-by-step explanation:
We are given with,
- Volume of Solution= 0.25 L
- Given mass of NaOH = 12 g
Molarity is defined as the number of moles of solute dissolved per litre of solution
Molarity of a solution is calculated by using formula:

Molar mass of NaOH = 40 g
Calculation for Number of moles:


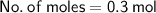
Hence, The number of moles of solute is 0.3 mol.
Calculating the Molarity:
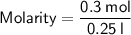
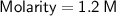
Hence, the molarity of the given solution solution is 1.2 M