A.
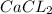 → Ca²⁺ + 2Cl⁻ + Energy
→ Ca²⁺ + 2Cl⁻ + Energy
B. Energy + CaCl2 → Ca²⁺ + 2Cl⁻
C.
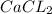 → Ca²⁺ + 2Cl⁻ ΔH = + energy
→ Ca²⁺ + 2Cl⁻ ΔH = + energy
The dissolving of calcium chloride is an exothermic process. Which reaction shows the thermochemical equation for the dissociation of CaCI2?
A. CaCl2 → Ca²⁺ + 2Cl⁻ + Energy
B. Energy + CaCl2 → Ca²⁺ + 2Cl⁻
C. CaCl2 → Ca²⁺ + 2Cl⁻ ΔH = + energy
Answer:
The correct answer is **A. CaCl2 → Ca²⁺ + 2Cl⁻ + Energy**.
In an exothermic reaction, heat is released, so it is shown as a product in the thermochemical equation. Option A is the only option that shows heat as a product.
Options B and C are incorrect because they show heat as a reactant. This would mean that the reaction requires heat to occur, which is not the case for an exothermic reaction.
Option C is also incorrect because the ΔH value is positive. A positive ΔH value means that the reaction is endothermic, not exothermic.
Therefore, the correct thermochemical equation for the dissociation of CaCI2 is A.
Here is a breakdown of the equation:
* **CaCl2** is the reactant, which is calcium chloride in the solid state.
* **Ca²⁺** and **2Cl⁻** are the products, which are calcium ions and chloride ions in the aqueous state.
* **Energy** is the product, which is the heat that is released when the reaction occurs.
* **ΔH < 0** indicates that the reaction is exothermic.