69.23 mL of the diluted solution should be administered to supply a dose of 52 mg.
How to get what has to be administered
First, find the amount of ketamine in the 3.6 mL of the original solution:
Amount of ketamine in 3.6 mL
= concentration of ketamine × volume of solution
Amount = 100 mg/mL × 3.6 mL
= 360 mg
a dose of 52 mg
Let x represent the amount of diluted solution needed to supply 52 mg of ketamine.
Using the concept of a constant amount of drug:
we can set up a proportion:
Amount of ketamine in 3.6 mL / Volume of diluted solution = Amount of ketamine needed for the dose / Total volume after dilution
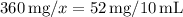
Now, solve for x:
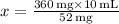
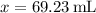
Therefore, approximately 69.23 mL of the diluted solution should be administered to supply a dose of 52 mg.