Answer:
Approximately
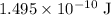 .
.
Step-by-step explanation:
Atomic mass unit (
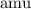 ) is a unit for mass. In standard unit, one atomic mass unit is equivalent to approximately
) is a unit for mass. In standard unit, one atomic mass unit is equivalent to approximately
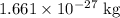 .
.
Substitute
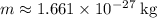 and
and
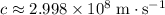 into the equation:
into the equation:
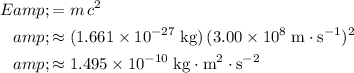 .
.
Note that
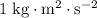 is equivalent to
is equivalent to
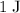 (one Joule.) Therefore, the energy equivalent to
(one Joule.) Therefore, the energy equivalent to
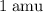 would be approximately
would be approximately
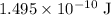 .
.