Final answer:
To calculate the heat released by the combustion of 13.2 kg of propane, we convert the mass to moles and then multiply by the heat of combustion per mole. This yields a total of 611,825.2 kJ of heat released during the complete combustion of the propane in the tank.
Step-by-step explanation:
To calculate the heat associated with the complete combustion of all the propane in the tank, we will use the heat of combustion of propane which is given as − 2044 kJ/mol. First, we need to find the number of moles of propane in 13.2 kg of propane.
Propane has a molecular weight of
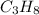 which is 3(12.01) + 8(1.008) = 44.096 g/mol. To convert kilograms to grams, multiply by 1000, so we have 13.2 * 1000 = 13200 g of propane. The number of moles of propane is then 13200 g ÷ 44.096 g/mol = 299.3 moles of propane.
which is 3(12.01) + 8(1.008) = 44.096 g/mol. To convert kilograms to grams, multiply by 1000, so we have 13.2 * 1000 = 13200 g of propane. The number of moles of propane is then 13200 g ÷ 44.096 g/mol = 299.3 moles of propane.
The total heat of combustion can be calculated by multiplying the number of moles of propane by the heat of combustion per mole:
Total heat = 299.3 moles * (− 2044 kJ/mol) = − 611,825.2 kJ
Since the heat of combustion is exothermic, it is released, and we can drop the negative sign to express that 611,825.2 kJ of heat is released.