The mass of CaCO3 consumed to produce the CO2 was approximately 58.91 milligrams.
To solve this two-part problem, we'll perform calculations based on the chemical reaction provided and the Ideal Gas Law for the conditions given. Here are the steps:
Part 1: Calculate the Moles of CO2 Produced
Step 1: Convert the Given Volume to Liters
- Since volume is given in milliliters, we need to convert it to liters because the Ideal Gas Law uses volume in liters.
![\[ 15.0 \text{ mL} = 15.0 * 10^(-3) \text{ L} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/2l00tk9kjf2qa0fad3butg6plx3qqnazpy.png)
Step 2: Convert Pressure to Atmospheres
- The Ideal Gas Law requires pressure in atmospheres. Convert the given pressure from mmHg to atmospheres.
![\[ 730.0 \text{ mmHg} * \frac{1 \text{ atm}}{760 \text{ mmHg}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bnav6lvbiu30k4vhtswnb5ghtm9n7uaryw.png)
Step 3: Convert Temperature to Kelvin
- The Ideal Gas Law requires temperature in Kelvin.
![\[ 25.0°C = 25.0 + 273.15 \text{ K} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/7teqnu5t8i3fd1ikymkamj55ez4r2us7ot.png)
Step 4: Use the Ideal Gas Law to Find Moles of CO2
- The Ideal Gas Law is
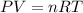 , where
, where
 is pressure,
is pressure,
 is volume,
is volume,
 is number of moles,
is number of moles,
 is the gas constant, and
is the gas constant, and
 is temperature.
is temperature.
- Solve for
 (number of moles of CO2).
(number of moles of CO2).
![\[ n = (PV)/(RT) \]](https://img.qammunity.org/2024/formulas/chemistry/college/c5r86em4utp4bl7j1182kn28nnim0yirm2.png)
Let's perform these calculations.
The number of moles of CO2 produced was approximately
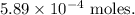
Part 2: Calculate the Milligrams of CaCO3 Consumed
Step 5: Determine the Molar Mass of CaCO3
- The molar mass of CaCO3 (calcium carbonate) is the sum of the atomic masses of its constituent atoms (Ca, C, and 3 O atoms).
Step 6: Calculate the Mass of CaCO3 Consumed
- Since the reaction between CaCO3 and H+ is a 1:1 molar ratio, the moles of CO2 produced will be the same as the moles of CaCO3 consumed.
- Convert the moles of CaCO3 to milligrams by multiplying with the molar mass of CaCO3 and then converting grams to milligrams by multiplying by 1000.
The mass of CaCO3 consumed to produce the CO2 was approximately 58.91 milligrams.