Answer:
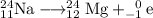
Explanation:
You want to identify the balanced nuclear reaction.
Balanced reaction
A balanced reaction will have the same total mass numbers and total atomic numbers on each side of the reaction. That is, the total of superscript numbers will be the same on both sides, and the total of subscript numbers will be the same on both sides.
A: 23 ≠ 24 + 1
B: 24 = 24 + 0 and 11 = 12 -1 . . . . balanced
C: 24 = 24 + 0 and 13 ≠ 12 -1
D: 23 ≠ 24 + 1
__
Additional comment
This reaction ejects an electron.
<95141404393>