Answer:
I will give up the electrons
Step-by-step explanation:
Consider the following rules:
1. An atom loses electrons if its oxidation number is positive.
2. An atom gains electrons if its oxidation number is negative.
3. An atom neither gains nor loses electrons if its atomic number is zero.
As I have an oxidation number
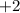 that is oxidation number is positive, so, I will give up electrons.
that is oxidation number is positive, so, I will give up electrons.