Final Answer:
The final concentration of iodide ions in the solution, after adding a 15.00 ml sample of the stock solution to 40.00 ml of water, is
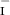 0.072 M.
0.072 M.
Step-by-step explanation:
In order to determine the final concentration of iodide ions, we can follow a step-by-step process. Firstly, let's find the moles of magnesium iodide in the stock solution. The molar mass of magnesium iodide
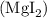 is calculated as follows:
is calculated as follows:
![\[\text{Molar mass of MgI}_2 = (\text{Atomic mass of Mg}) + 2 * (\text{Atomic mass of I}) = 24.31 + 2 * 126.90 \, \text{g/mol} = 277.11 \, \text{g/mol}\]](https://img.qammunity.org/2024/formulas/physics/high-school/ls26ia5x9iquhtp69q8q3ql2dmzkwmoa32.png)
Next, we find the moles of magnesium iodide in the stock solution:
![\[\text{Moles of MgI}_2 = \frac{\text{Mass}}{\text{Molar mass}} = \frac{4.68 \, \text{g}}{277.11 \, \text{g/mol}} \approx 0.0169 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/physics/high-school/lofvgbfoz508momhijzjvz6ahwg4ntbv01.png)
Now, let's calculate the concentration of iodide ions in the stock solution:
![\[\text{Concentration of }_\text{I}^- \text{ ions} = \frac{\text{Moles of }_\text{I}^-}{\text{Volume of solution}} = \frac{0.0169 \, \text{mol}}{0.075 \, \text{L}} \approx 0.225 \, \text{M}\]](https://img.qammunity.org/2024/formulas/physics/high-school/sq8hobzyxtcgjmn5g6mrmq50mf92lncb3v.png)
When a 15.00 ml sample is taken and added to 40.00 ml of water, the total volume becomes 55.00 ml. Now, we can find the final concentration:
![\[\text{Final concentration of }_\text{I}^- \text{ ions} = \frac{\text{Initial concentration} * \text{Initial volume}}{\text{Final volume}} = \frac{0.225 \, \text{M} * 15.00 * 10^(-3) \, \text{L}}{55.00 * 10^(-3) \, \text{L}} \approx 0.072 \, \text{M}\]](https://img.qammunity.org/2024/formulas/physics/high-school/dhx80xy07pjtcv3705stoasmtj9uhsd268.png)
Therefore, the final concentration of iodide ions is
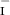 0.072 M.
0.072 M.