Answer:
Step-by-step explanation:
Calculation for number of moles-
To calculate the number of moles of any substance in the sample, we can simply divide the given weight of the substance by its molar mass.
Formula for Calculation for number of moles -
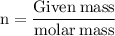
In this question, We have been given 9.019 g sample of ammonia i.e Given mass of ammonia is 9.019 g and the molar mass is 17.030 g/mol.
Plugging the values in above formula:
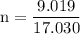
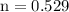
Hence, 0.529 mol of ammonia is present in 9.019 g sample of ammonia