Answer:
c) Silicon
Step-by-step explanation:
The easiest way to identify the element from a given Electronic Configuration is by observing the last subshell.
The Principal Quantum Number of the subshell will be your period number and the valence number of electrons (the number of electrons filled in the last subshell) will give you your Group from that particular block (s,p,d,f) . Here, 'p'.
Usually, For any subshell:
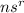 , where s is any subshell (not necessarily 's'),
, where s is any subshell (not necessarily 's'),
'n' is it's Principal Quantum Number
'r' is the number electrons in the subshell.
In this case,
We are given the electron configuration as:
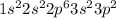
Observing the last subshell:
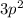
We find that,
The Principal Quantum Number is 3 and the number of valence electrons in that subshell is 2. Hence, we need to check for an element which has a Period Number 3 and since the last subshell is p. We need to check the second group of 'p' block. From any Periodic Table, we know that p-block starts from group 13. Hence, we need to check the 3rd element of group 14th since 14 is 2 groups from 13 (We need to count 13 too). Hence, the 3rd period element of the 14th group is 'Silicon'.