To calculate the theoretical yield and the percent yield for the reaction between aluminum (Al) and ozone (O3), we first need to balance the chemical equation and then construct a Before-Change-After (BCA) table.
Following this, we can calculate the theoretical yield and the percent yield.
Let's break it down step by step:
Step 1: Balance the Chemical Equation
The balanced chemical equation is:
![\[ 2 \text{Al} + 3 \text{O}_3 \rightarrow \text{Al}_2\text{O}_3 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ixqt8fx86rvg0xp5pzl5gyw3vqrmdkro80.png)
Step 2: Construct a BCA Table
To construct a BCA table, we need the initial amounts of reactants. Since you haven't provided specific amounts of aluminum and ozone, I'll use hypothetical amounts for the purpose of this example. Let's assume we start with 1.00 mole of Al and 1.50 moles of O3.
| Reactant/Product | Initial (moles) | Change (moles) | Final (moles) |
|------------------|-----------------|----------------|---------------|
| Al | 1.00 | -1.00 | 0 |
| O3 | 1.50 | -1.50 | 0 |
| Al2O3 | 0 | +0.50 | 0.50 |
Step 3: Calculate Theoretical Yield
The theoretical yield is determined by the limiting reactant. In this case, both reactants are completely consumed, so either could be considered the limiting reactant. The moles of Al2O3 formed (0.50 moles) is our theoretical yield in moles.
To convert this to grams, we use the molar mass of Al2O3:
![\[ \text{Molar mass of Al}_2\text{O}_3 = 2 * 26.98 (\text{Al}) + 3 * 16.00 (\text{O}) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/89xpff4nruaro0ed2fsowi1v79ophr0jcf.png)
Let's calculate the molar mass and then the theoretical yield in grams.
Step 4: Calculate Percent Yield
To calculate the percent yield, we need the actual yield (the amount of Al2O3 actually produced in grams). Since this information is not provided, I will use a hypothetical actual yield. Let's assume the actual yield is 40 grams.
The percent yield is calculated as:
![\[ \text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) * 100\% \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jrez201hq270rh3vbqad64pj9ojgwebrx8.png)
Now, let's do the calculations for the molar mass of Al2O3 and the theoretical yield in grams.
The molar mass of Aluminum Oxide (Al₂O₃) is approximately 101.96 g/mol. Based on our BCA table, the theoretical yield for the reaction is about 50.98 grams of Al₂O₃.
Given the hypothetical actual yield of 40.0 grams of Al₂O₃, the percent yield of the reaction can be calculated as approximately 78.46%.
To summarize:
- Molar Mass of Al₂O₃:
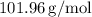
- Theoretical Yield:

- Percent Yield:
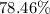
This calculation assumes the initial amounts of aluminum and ozone as 1.00 mole and 1.50 moles respectively.