Answer:
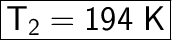
Step-by-step explanation:
Given data:
Initial Volume = V1 = 800 mL
Initial Temperature = T1 = 115 + 273 = 388 K
Final Volume = V2 = 400 mL
Required:
Final Temperature = T2 = ?
Formula:
 (Charles Law)
(Charles Law)
Solution:
Put the given data in the above formula.
![\displaystyle (800)/(388) = (400)/(T_2) \\\\Cross \ Multiply.\\\\800 * T_2 = 400 * 388\\\\800 * T_2 = 155200\\\\Divide \ both \ sides \ by \ 800.\\\\T_2 = (155200)/(800) \\\\T_2 = 194 \ K \\\\\rule[225]{225}{2}](https://img.qammunity.org/2024/formulas/chemistry/high-school/69819pmzlunhhn8wnm2vn3a9hzfvwsfv5a.png)