Step-by-step explanation:
first you need to know what's the reaction that's taking place
h2so4 + 2 naoh -> na2so4 + 2 h2o
from this equation you know that 1mol h2so4 neutralises 2 mol naoh, so there's a relation that you can use as follows
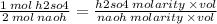
rearranging the terms you get
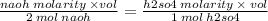
naoh molarity is
20g/L × 1mol/40g = 0.5 mol / L
using the data you have, in the formula
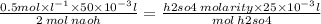
you get that
h2so4 molarity = 0.5 mol/L
if h2so4 molar mass is 98g/mol, then
(0.5mol / L) × (98g/mol) = 49g/L
then the concentration of the sulfuric acid in grams per dm3 is 49g/dm³