Answer:
Approximately
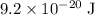 assuming that both particles behave like point charges.
assuming that both particles behave like point charges.
Step-by-step explanation:
In a system consisting of two point charges
 and
and
 at a distance of
at a distance of
 apart from each other, the electrical potential energy of the system would be:
apart from each other, the electrical potential energy of the system would be:
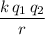 ,
,
Where
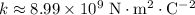 is the Coulomb Constant.
is the Coulomb Constant.
The electric charge on each proton is equal to the elementary charge: approximately
 . Electric charge on each electron has the same magnitude but the opposite sign: approximately
. Electric charge on each electron has the same magnitude but the opposite sign: approximately
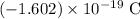
Therefore, in this question, the two charges in the system are:
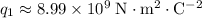 for the proton, and
for the proton, and
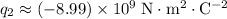 .
.
Apply unit conversion and ensure that the distance between the two particles is in the standard unit of meters:
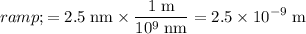 .
.
Substitute these values into the equation for the electric potential:
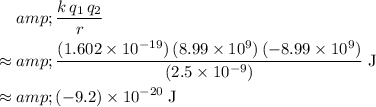 .
.
In other words, the electric potential of this system would be approximately
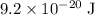 .
.