You have created approximately 137.4 J/K of new entropy by mixing the hot and cold water.
How to find new entropy?
Calculating the Change in Entropy
To calculate the change in entropy (ΔS) when mixing the hot and cold water, we can use the following formula:
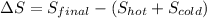
where:
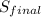 = total entropy of the mixture
= total entropy of the mixture
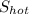 = entropy of the hot water
= entropy of the hot water
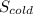 = entropy of the cold water
= entropy of the cold water
Use the formula for the entropy of an ideal gas:
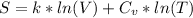
where:
k = Boltzmann's constant (1.38 × 10^-23 J/K)
V = volume of the water
 = heat capacity at constant volume (4.184 J/g°C for water)
= heat capacity at constant volume (4.184 J/g°C for water)
T = temperature in Kelvin (K)
For the hot water:
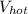 = 50 liters
= 50 liters
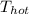 = 55°C + 273.15 = 328.15 K
= 55°C + 273.15 = 328.15 K
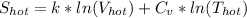
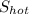 = 1.38 × 10⁻²³ J/K × ln(50 liters) + 4.184 J/g°C × ln(328.15 K)
= 1.38 × 10⁻²³ J/K × ln(50 liters) + 4.184 J/g°C × ln(328.15 K)
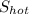 = 658.7 J/K
= 658.7 J/K
For the cold water:
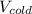 = 25 liters
= 25 liters
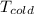 = 10°C + 273.15 = 283.15 K
= 10°C + 273.15 = 283.15 K
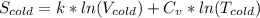
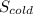 = 1.38 × 10⁻²³ J/K × ln(25 liters) + 4.184 J/g°C × ln(283.15 K)
= 1.38 × 10⁻²³ J/K × ln(25 liters) + 4.184 J/g°C × ln(283.15 K)
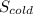 = 593.1 J/K
= 593.1 J/K
Calculate the total entropy of the mixture:
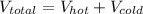
= 50 liters + 25 liters
= 75 liters
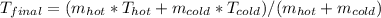
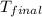 = (50 kg × 55°C + 25 kg × 10°C) / (50 kg + 25 kg)
= (50 kg × 55°C + 25 kg × 10°C) / (50 kg + 25 kg)
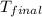 = 38.33°C
= 38.33°C
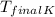 = 38.33°C + 273.15
= 38.33°C + 273.15
= 311.48 K
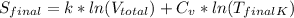
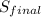 = 1.38 × 10⁻²³ J/K × ln(75 liters) + 4.184 J/g°C × ln(311.48 K)
= 1.38 × 10⁻²³ J/K × ln(75 liters) + 4.184 J/g°C × ln(311.48 K)
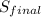 = 789.2 J/K
= 789.2 J/K
Calculate the change in entropy:
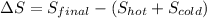
ΔS = 789.2 J/K - (658.7 J/K + 593.1 J/K)
ΔS ≈ 137.4 J/K
Therefore, you have created approximately 137.4 J/K of new entropy by mixing the hot and cold water.