The required molarity of the resulting solution is :
Step-by-step explanation:
Molarity is defined as the number of moles of solute dissolved per litre of solution.
Formula :
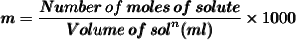
Here we are given .
- Mass of NaOH = 0.38 g
- Molar mass of NaOH = 40 g
No. of moles = Given mass/Molar Mass
- No. of moles = 0.38/40
- No. of moles = 0.02 g
- Volume of solution= 50 ml
So Simply by plugging in the values of moles of solute and volume of solution in the above formula we will get the required Molarity of the resulting solution..
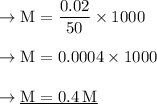
So The required Molarity of the resulting solution is 0.4 M