To solve this problem, we can use the given density of acetone and convert the mass from pounds to grams.
First, let's convert the mass from pounds to kilograms:
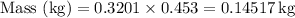
Now, we can use the density formula to find the volume in cubic centimeters (cm³):
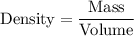
Rearranging the formula to solve for volume, we have:
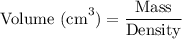
Substituting the given values, we get:
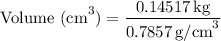
Now, let's convert the volume from cubic centimeters to milliliters:
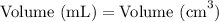
Substituting the value we obtained earlier, we have:
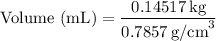
Calculating the value, we find:
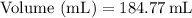
Therefore, the volume of acetone would be approximately 184.8 mL.
The correct option is A. 184.8 mL.
♥️