The vapor pressure of a solution made by mixing two volatile liquids (liquids that easily evaporate) can be calculated using Raoult's Law, which states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component multiplied by its mole fraction in the mixture.
First, we need to calculate the mole fraction of each component. The mole fraction is the ratio of the number of moles of a component to the total number of moles of all components in the mixture.
The number of moles of a substance can be calculated using the formula:
n = mass / molar mass
The molar mass of ethanol (C2H6O) is approximately 46.07 g/mol, and the molar mass of water (H2O) is approximately 18.02 g/mol.
First, let's calculate the mass of each component using the given volumes and densities, then calculate the number of moles of each component, and finally calculate the mole fractions. We'll use WolframAlpha to do these calculations.
The mole fraction of ethanol,
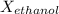 , is approximately 0.1427 and the mole fraction of water,
, is approximately 0.1427 and the mole fraction of water,
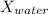 , is approximately 0.8573.
, is approximately 0.8573.
Now we can calculate the vapor pressure of the solution using Raoult's Law, which states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component multiplied by its mole fraction in the mixture.
The vapor pressure of the solution,
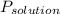 , is given by:
, is given by:
![\[P_(solution) = X_(ethanol) * P_(ethanol) + X_(water) * P_(water)\]](https://img.qammunity.org/2024/formulas/chemistry/college/8cawjbww2g2mu4fdcis0bkgoq2d8s30q80.png)
where
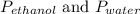 are the vapor pressures of pure ethanol and water, respectively. Given in the problem,
are the vapor pressures of pure ethanol and water, respectively. Given in the problem,
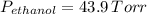 and
and
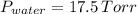 .
.
The vapor pressure of the solution at 20 °C is approximately 21.27 Torr. This is calculated using Raoult's Law, taking into account the mole fractions of ethanol and water and the vapor pressures of pure ethanol and water.