Answer: The energy of one quantum of radiation having a wavelength of
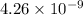 meters is greater than the energy of one photon of orange light.
meters is greater than the energy of one photon of orange light.
Step-by-step explanation:
The energy of a photon or a quantum of radiation can be calculated using the formula:
E = hc/λ
where:
- E is the energy of the photon,
- h is Planck's constant (6.626 x 10^-34 J*s),
- c is the speed of light (3.0 x 10^8 m/s), and
- λ is the wavelength of the light.
The wavelength of orange light is typically around 600 nm (or 600 x 10^-9 m).
The energy of a photon of orange light, which has a wavelength of approximately 600 nm, is approximately
 Joules or 2.066 electronvolts (eV).
Joules or 2.066 electronvolts (eV).
The energy of a quantum of radiation with a wavelength of
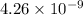 meters is approximately
meters is approximately
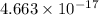 Joules or 29.1 electronvolts (eV).
Joules or 29.1 electronvolts (eV).