Final answer:
The claim made by the thermodynamicist is not valid because it violates the Second Law of Thermodynamics. The maximum theoretical efficiency of a heat engine operating between the given temperatures is approximately 59.6%.
Step-by-step explanation:
The claim made by the thermodynamicist is not valid because it violates the Second Law of Thermodynamics, which states that no heat engine can have an efficiency greater than the Carnot efficiency.
To assess the validity of the claim about the heat engine's thermal efficiency, we can use the formula for the thermal efficiency of a heat engine:
![\[ \text{Efficiency} = 1 - (T_C)/(T_H) \]](https://img.qammunity.org/2024/formulas/business/high-school/uo8ec2wcz1ogbmxv69dpaimoeymx9v0hvd.png)
Where:
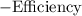 is the thermal efficiency of the engine.
is the thermal efficiency of the engine.
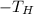 is the absolute temperature of the hot reservoir.
is the absolute temperature of the hot reservoir.
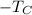 is the absolute temperature of the cold reservoir.
is the absolute temperature of the cold reservoir.
Given:
- The hot reservoir's absolute temperature
 .
.
- The cold reservoir's absolute temperature

Let's substitute these values into the formula to calculate the theoretical maximum efficiency:
![\[ \text{Efficiency} = 1 - (510)/(1260) \]](https://img.qammunity.org/2024/formulas/business/high-school/btdq13o0dlkja8jo0h56urfcghltf6hvof.png)
![\[ \text{Efficiency} = 1 - 0.404 \]](https://img.qammunity.org/2024/formulas/business/high-school/gr8qmivr343chom3a3obqhegki7r3l4uqp.png)
![\[ \text{Efficiency} = 0.596 \]](https://img.qammunity.org/2024/formulas/business/high-school/99lm7t0kyh3kki43xwbpva1z0tlqd6p2zl.png)
Thus, the maximum theoretical efficiency of a heat engine operating between these two temperatures is approximately 59.6%.
Since the claimed efficiency of 50% is lower than the maximum theoretical efficiency, the claim is not valid.