The atomic mass of Scandium (^45Sc) is approximately 44.955907 unified atomic mass units (u), or equivalently 45 g/mol.
To find the number of atoms in the given sample, we can use the formula:
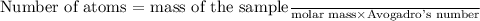
Given that the mass of the sample is 7.8211 g and Avogadro's number is approximately
 atoms/mol, we can substitute these values into the formula to find the number of atoms.
atoms/mol, we can substitute these values into the formula to find the number of atoms.
Once we have the number of atoms, we can determine the number of protons, neutrons, and electrons. For ^45Sc:
- The number of protons is equal to the atomic number of Scandium, which is 21.
- The number of neutrons is equal to the mass number minus the atomic number, which is 45 - 21 = 24.
- The number of electrons in a neutral atom is equal to the number of protons, so there are also 21 electrons.
Let's calculate the number of atoms first.
The number of atoms in the 7.8211 g sample of Scandium (^45Sc) is approximately
 .
.
Given that each atom of Scandium has:
- 21 protons
- 24 neutrons
- 21 electrons (in a neutral atom)
We can multiply these numbers by the total number of atoms to get the total number of each particle in the sample. Let's calculate that.
In the 7.8211 g sample of Scandium (^45Sc), there are approximately:
-
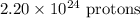
-
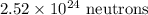
-
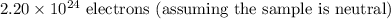
Please note that these are approximate values.