Answer:
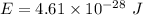
Step-by-step explanation:
Given that,
The frequency of local AM radio station, f = 696 KHz = 696000 Hz
We need to find the energy of the frequency at which it is broadcasting.
We know that,
Energy of a wave, E = hf
Where
h is Planck's constant
Put all the values,
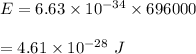
So, the energy of the wave is equal to
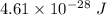 .
.