* Tantalum forms a face-centered cubic (fcc) unit cell with 4 atoms per unit cell.
* The density of vanadium is 5.97 g/cm^3.
The density of tantalum is 16.6 g/cm^3. The edge length of the unit cell is 330 pm. The molar mass of tantalum is 181 g/mol. The Avogadro constant is 6.022 * 10^23 mol^-1.
The density of a substance is defined as the mass per unit volume. So, the density of tantalum can be calculated as follows:
density = mass / volume
density = 1
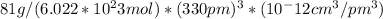
density = 16.6 g/cm^3
The number of atoms per unit cell can be calculated as follows:
number of atoms = density * volume / molar mass * Avogadro constant
number of atoms =
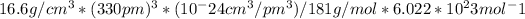
number of atoms = 4
Therefore, tantalum forms a face-centered cubic (fcc) unit cell with 4 atoms per unit cell.
The density of vanadium can be calculated as follows:
density =
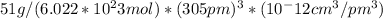
density = 5.97 g/cm^3
Therefore, the density of vanadium is 5.97 g/cm^3.