Answer:
A ball of zinc.
Explanation:
Density is defined as the ratio of an object's mass to its volume.
The formula for density is:
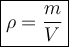
where:
- ρ is density measured in kilograms per cubic metre (g/cm³).
- m is mass measured in grams (g).
- V is volume measured in cubic centimeters (cm³).
Therefore, to calculate the weight of each ball, multiply its volume by the density of the material.
A ball can be modelled as a sphere. Therefore, calculate the volumes of the balls by using the formula for the volume of a sphere.

Substitute the respective values of r into the formula to calculate the volumes of the two balls.


The density of zinc (Zn) is approximately 7.14 g/cm³.
The density of chromium (Cr) is approximately 7.15 g/cm³.
Therefore, the approximate weights of the two balls are:
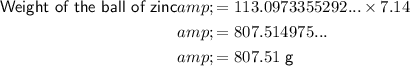
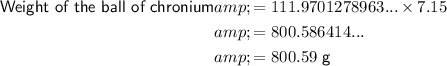
As 807.51 > 800.59, a ball of zinc with a radius of 3 cm weighs more than a ball of chromium with a radius of 2.99 cm.