The work function of the metal is 3.13 *
 J
J
The work function is commonly expressed in electron volts (eV) or joules (J) and depends on the material's properties. Different materials have different work functions, and this parameter plays a crucial role in understanding phenomena like the photoelectric effect and electron emission from surfaces.
The energy of the photon is
E = hf
E = 6.6 *
 * 1.05 *
* 1.05 *

= 6.93 *
 J
J
W = E - KE
W = 6.93 *
 -
-
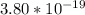
W = 3.13 *
 J
J
Missing parts;
When a metal was exposed to photons at a frequency of 1.05×1015 s−1, electrons were emitted with a maximum kinetic energy of 3.80×10−19 J.
Calculate the work function, Φ, of this metal.