Answer:
The half equations for the electrolysis of aluminium oxide are:
Cathode:
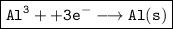
Anode:
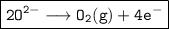
1. Aluminium oxide has a very high melting point (2050°C). By dissolving it in molten cryolite (melting point 960°C), the melting point of the mixture is lowered to 950°C. This makes it easier to melt and transport the aluminium oxide, and also reduces the amount of energy required for electrolysis.
2. The carbon anodes are oxidized by the oxygen gas produced at the anode, forming carbon dioxide. This reaction is:

The carbon anodes need to be replaced regularly because they are gradually consumed by this reaction. This adds to the cost of aluminium extraction.