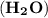Answer:
The chemical equations for the reactions you mentioned are:
a) Aluminium oxide reacting with sodium hydroxide

In this reaction, aluminium oxide
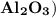 reacts with sodium hydroxide (NaOH) to form sodium aluminate
reacts with sodium hydroxide (NaOH) to form sodium aluminate
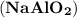 and water
and water
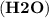
b) Action of
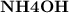 on
on
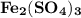
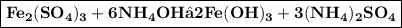
In this reaction, iron (III) sulfate
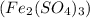 reacts with ammonium hydroxide
reacts with ammonium hydroxide
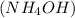 to form iron (III) hydroxide
to form iron (III) hydroxide
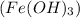 and ammonium sulfate
and ammonium sulfate
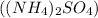 .
.
c) Zinc oxide is treated with sodium hydroxide solution
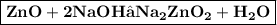
In this reaction, zinc oxide (ZnO) reacts with sodium hydroxide (NaOH) to form sodium zincate (Na_2ZnO_2) and water